Is Acetone More Polar Than Propanol . Yes, propanol is more polar than acetone because it has a longer carbon chain which allows for more hydrophobic interactions. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl would be more soluble in acetone than in. Common solvents arranged from the least polar to the most polar It is critical in the jones oxidation. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. A variety of organic reactions employ acetone as a polar, aprotic solvent. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because acetone is cheap, volatile, and dissolves or decomposes with. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane.
from www.numerade.com
23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. It is critical in the jones oxidation. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl would be more soluble in acetone than in. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. A variety of organic reactions employ acetone as a polar, aprotic solvent. Common solvents arranged from the least polar to the most polar Yes, propanol is more polar than acetone because it has a longer carbon chain which allows for more hydrophobic interactions. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because acetone is cheap, volatile, and dissolves or decomposes with.
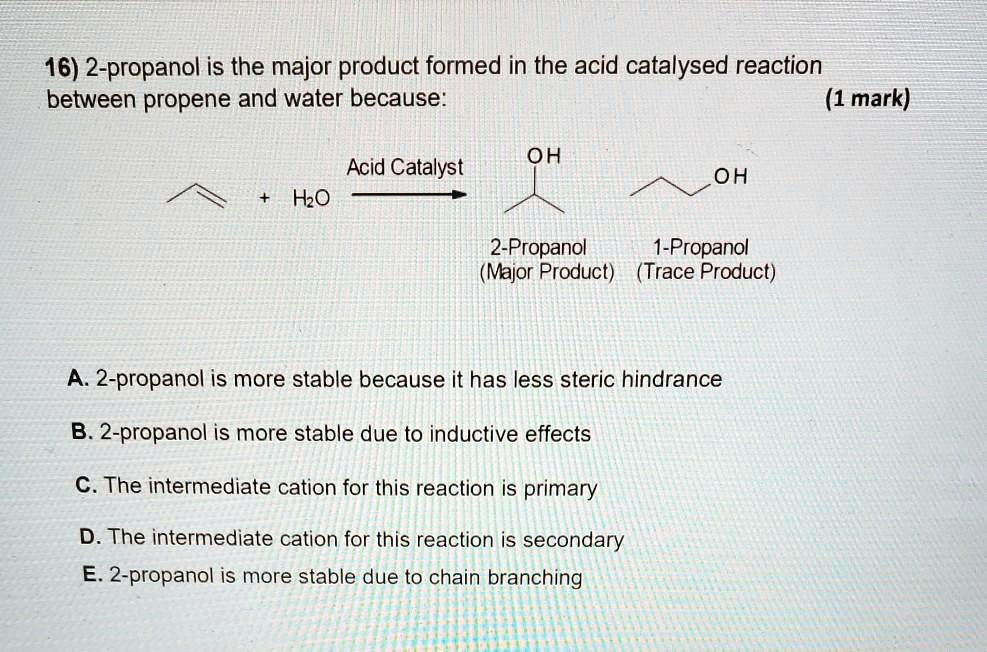
SOLVED 16) 2propanol is the major product formed in the acid
Is Acetone More Polar Than Propanol A variety of organic reactions employ acetone as a polar, aprotic solvent. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because acetone is cheap, volatile, and dissolves or decomposes with. A variety of organic reactions employ acetone as a polar, aprotic solvent. Common solvents arranged from the least polar to the most polar Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl would be more soluble in acetone than in. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Yes, propanol is more polar than acetone because it has a longer carbon chain which allows for more hydrophobic interactions. It is critical in the jones oxidation.
From passmyexams.co.uk
Oxidation of Propanol Easy exam revision notes for GSCE Chemistry Is Acetone More Polar Than Propanol 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. It is critical in the jones oxidation. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water. Is Acetone More Polar Than Propanol.
From magdalenakruwsalazar.blogspot.com
Acetone Polar or Nonpolar Solvent MagdalenakruwSalazar Is Acetone More Polar Than Propanol It is critical in the jones oxidation. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Common solvents arranged from the least polar to the most polar Because acetone is cheap, volatile, and dissolves or decomposes. Is Acetone More Polar Than Propanol.
From www.numerade.com
SOLVED CaHeo a) Propanone (acetone) and propanal (propionaldehyde) are Is Acetone More Polar Than Propanol Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl would be more soluble in acetone than in. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Common solvents arranged from. Is Acetone More Polar Than Propanol.
From www.numerade.com
SOLVED Question 5 The reduction of propanone (acetone) with hydrogen Is Acetone More Polar Than Propanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Because acetone is cheap, volatile, and dissolves or decomposes with. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that. Is Acetone More Polar Than Propanol.
From courses.lumenlearning.com
Properties of Liquids Chemistry for Majors Is Acetone More Polar Than Propanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because acetone is cheap, volatile, and dissolves or decomposes with. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl would be more soluble in acetone than in. It is critical in the. Is Acetone More Polar Than Propanol.
From www.vedantu.com
The most reactive of the following is A Acetone B Benzophenone class 11 Is Acetone More Polar Than Propanol Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because acetone is cheap, volatile, and dissolves or decomposes with. A variety of organic reactions employ acetone as a polar, aprotic solvent. Common solvents arranged from the least polar to the most polar 23 rows however, acetone is still. Is Acetone More Polar Than Propanol.
From www.numerade.com
SOLVED Table Relative polarity of common mobile phases Acetic acid Is Acetone More Polar Than Propanol It is critical in the jones oxidation. Common solvents arranged from the least polar to the most polar Because acetone is cheap, volatile, and dissolves or decomposes with. Yes, propanol is more polar than acetone because it has a longer carbon chain which allows for more hydrophobic interactions. A variety of organic reactions employ acetone as a polar, aprotic solvent.. Is Acetone More Polar Than Propanol.
From www.doubtnut.com
Explain why propanol has a higher boiling point than hydrocarbon butan Is Acetone More Polar Than Propanol A variety of organic reactions employ acetone as a polar, aprotic solvent. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl would be more soluble in acetone than in. It is critical in the jones oxidation. Water acetic acid ethylene glycol methanol. Is Acetone More Polar Than Propanol.
From www.slideshare.net
Solvents used in pharmacy Is Acetone More Polar Than Propanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl would be more soluble in acetone than in. Common solvents arranged from the least polar to the most polar 23 rows however, acetone. Is Acetone More Polar Than Propanol.
From www.numerade.com
SOLVED'1)Put the following chromatography solvent in order of polarity Is Acetone More Polar Than Propanol Common solvents arranged from the least polar to the most polar Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl would be more soluble in acetone than in. It is critical. Is Acetone More Polar Than Propanol.
From www.numerade.com
SOLVED Part 3. For each of the following pairs of compounds, determine Is Acetone More Polar Than Propanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. A variety of organic reactions employ acetone as a polar, aprotic solvent. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because acetone is cheap, volatile, and dissolves or decomposes with. Common solvents arranged from the least polar to the most polar Because the dipole moment of acetone. Is Acetone More Polar Than Propanol.
From www.makethebrainhappy.com
MakeTheBrainHappy Is Acetone Polar or Nonpolar? Is Acetone More Polar Than Propanol Common solvents arranged from the least polar to the most polar Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl would be more soluble in acetone than in. Because acetone is. Is Acetone More Polar Than Propanol.
From www.gizmoplans.com
Is Acetone Polar? Properties, Uses, Molecular Structure Is Acetone More Polar Than Propanol 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. A variety of organic reactions employ acetone as a polar, aprotic solvent. It is critical in. Is Acetone More Polar Than Propanol.
From jimmywhittle.blogspot.com
Intermolecular Forces Acetone (CH3)2CO Is Acetone More Polar Than Propanol Common solvents arranged from the least polar to the most polar A variety of organic reactions employ acetone as a polar, aprotic solvent. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that. Is Acetone More Polar Than Propanol.
From getrevising.co.uk
GCSE Chemistry C3 Organic Chemistry Revision Cards in GCSE Chemistry Is Acetone More Polar Than Propanol Yes, propanol is more polar than acetone because it has a longer carbon chain which allows for more hydrophobic interactions. It is critical in the jones oxidation. Common solvents arranged from the least polar to the most polar A variety of organic reactions employ acetone as a polar, aprotic solvent. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile. Is Acetone More Polar Than Propanol.
From www.researchgate.net
Experimental breakthrough times of polar VOCs (acetone, 2propanol) and Is Acetone More Polar Than Propanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl. Is Acetone More Polar Than Propanol.
From www.numerade.com
SOLVED 16) 2propanol is the major product formed in the acid Is Acetone More Polar Than Propanol 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. It is critical in the jones oxidation. A variety of organic reactions employ acetone as a polar, aprotic solvent. Yes, propanol is more polar than acetone because it has a longer carbon chain which allows for more. Is Acetone More Polar Than Propanol.
From www.chegg.com
Solved cyclobutanol or propanol which one is more polar? A Is Acetone More Polar Than Propanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because acetone is cheap, volatile, and dissolves or decomposes with. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl would be. Is Acetone More Polar Than Propanol.